
- #Solution precipitate calculator how to
- #Solution precipitate calculator full
- #Solution precipitate calculator series
#Solution precipitate calculator series
For those tests, a series of compounds can be added to deduce what ions are present. Additionally, these tests are also commonly used in chemistry labs. The lead would precipitate out as either PbCl 2 or Pb(OH) 2 and indicate that lead is present. For example, to determine if lead (Pb 2+) is present in the solution, a solution containing chlorides or hydroxides could be added. Precipitation reactions are commonly used to identify if certain ions are present in a solution.
#Solution precipitate calculator how to
To learn how to name these compounds, read the Naming Ionic Compounds tutorial! Uses of Precipitation Reactions and Real-Life Examples
#Solution precipitate calculator full
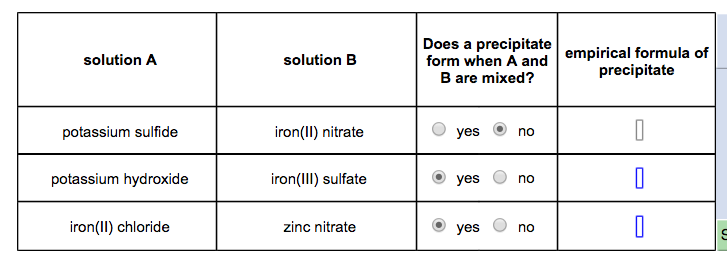
Carbonates, chromates, phosphates, and fluorides are all insoluble except for with alkali metals and ammonium.Ī full solubility chart and description of what compounds are soluble can be found on the Solubility Rules tutorial.Sulfides are insoluble except with the alkali and alkaline earth metals.Hydroxides are slightly soluble with the alkaline earth metals. Hydroxides are insoluble except for with alkali metals.Sulfates are soluble, except with Ca 2+, Sr 2+, Ba 2+, Pb 2+, and Ag 2+.The two main exceptions are silver acetate and silver nitrate. Chlorides, bromides, and iodides are soluble except when with Ag +, Pb 2+, and Hg 2 2+.Nitrates, acetates, chlorates, and perchlorates are generally soluble.There are also some general rules you can learn for the solubility of different compounds. A great resource is finding a good solubility table or solubility chart. These are general guidelines or rules on what compounds will form a precipitate. Both the charge and the number of atoms of each element must be balanced.

Net ionic equations must be balanced to be accurate. What is left is called the net ionic equation. These ions are also called spectator ions. To simplify this equation further, get rid of any ion that appears on both the reactant and product side as that indicates they are not part of the reaction. This format is called the complete ionic equation:Īg +(aq) + NO 3 –(aq) + Na +(aq) + Cl –(aq) –> AgCl(s) + Na +(aq) + NO 3 –(aq)
So the reaction including all components would be:ĪC(aq) + BD(aq) –> AD(s) + B +(aq) + C –(aq)ĪgNO 3(aq) + NaCl (aq) –> AgCl(s) + Na +(aq) + NO 3 –(aq)īecause the reactants are aqueous and we want to know the ions in solution, it is common to write the reaction in terms of the ions. The reactants must be ionic compounds in solution. The most general form of a precipitation reaction then is: The product must include a solid product. The reactants are usually two or more ionic aqueous molecules. How to Identify a Precipitation ReactionĪ precipitation reaction will always have a solid product. These reactions are commonly used to help determine what ions are in the solution. The liquid left behind is referred to as the supernatant. The solid particles can then also be removed from the solution by various means such as filtration, decantation, centrifuging. In precipitation reactions, the formed precipitate can remain suspended in solution or may sink to the bottom. AB and CD are both ionic compounds aqueous in solution. To be a precipitate reaction, either AD or CB will be an insoluble solid. This type of reaction takes the following form. The other pair of cation and anion may or may not be soluble.


That is when there are two salts that are soluble, and when the cation of one binds with the anion of the other it forms a solid product that is not soluble. Often, a precipitation reaction is a ‘double replacement reaction’. Lead iodide precipitating out of solution as a solid compound (from Wikipedia Commons)


 0 kommentar(er)
0 kommentar(er)
